
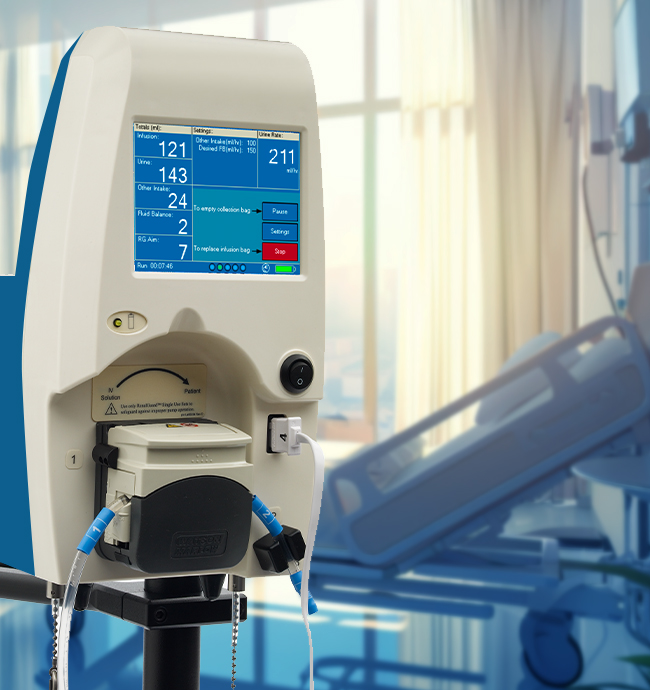
accurately balancing urine output with hydration

Real-time monitoring enables timely intervention

Seamless integrates into current workflow

FDA Breakthrough Device Designation

Validated in clinical study and endorsed